Favorite Info About How To Tell If An Atom Is Polar Or Nonpolar

Start by drawing its lewis structure.
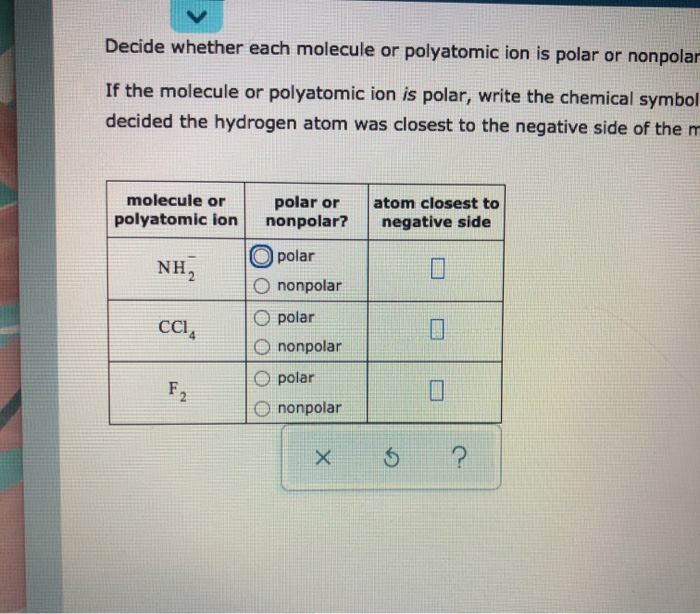
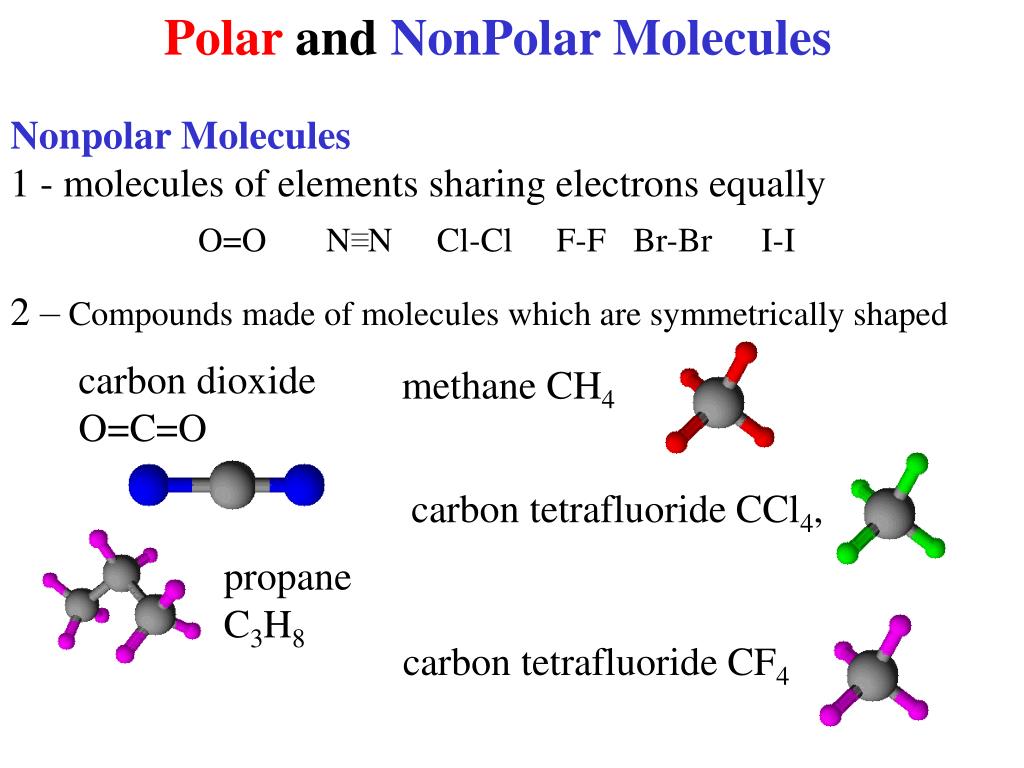
How to tell if an atom is polar or nonpolar. By the end of this section, you will be able to: Nonpolar compounds will be symmetric, meaning all of the. This rule applies to all molecules except hydrocarbons and molecules.
Examine each of the atomic elements contained within the molecule. A diatomic molecule that consists of a polar covalent. Polar bonds have a high melting point, surface tension,.
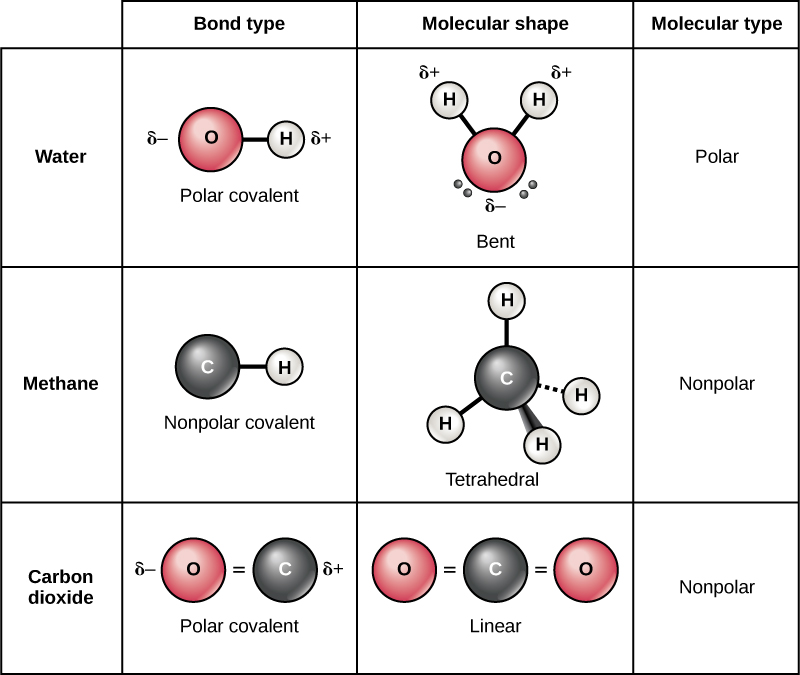
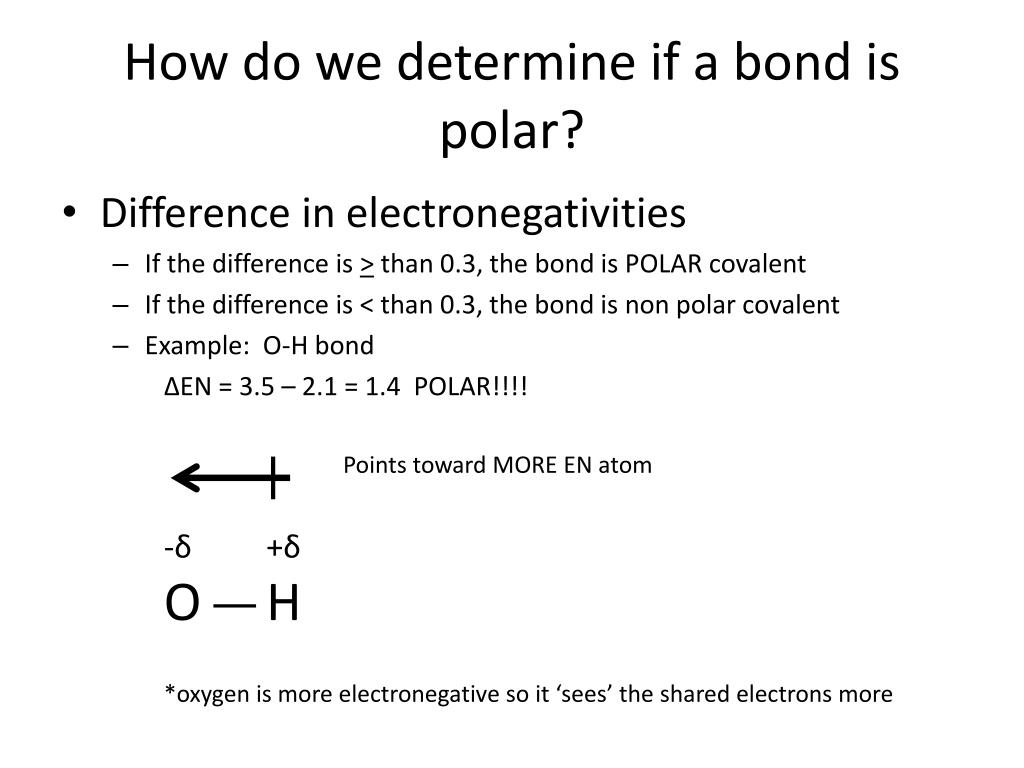
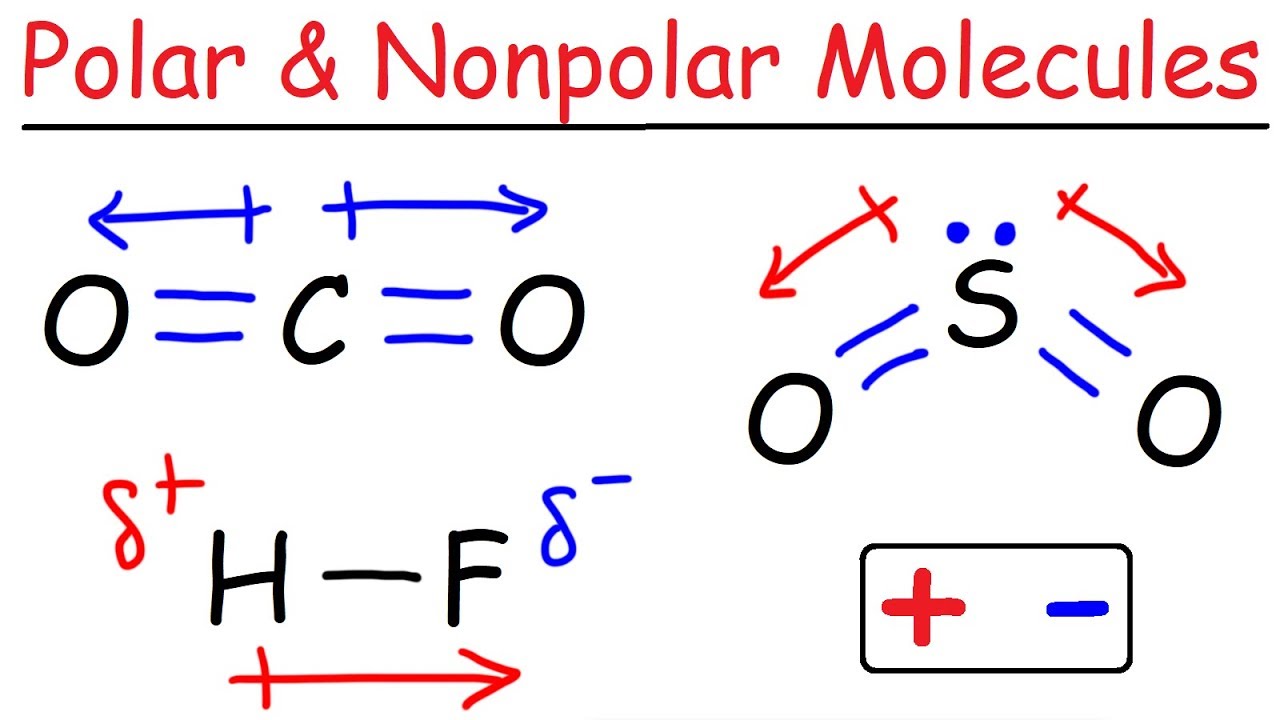
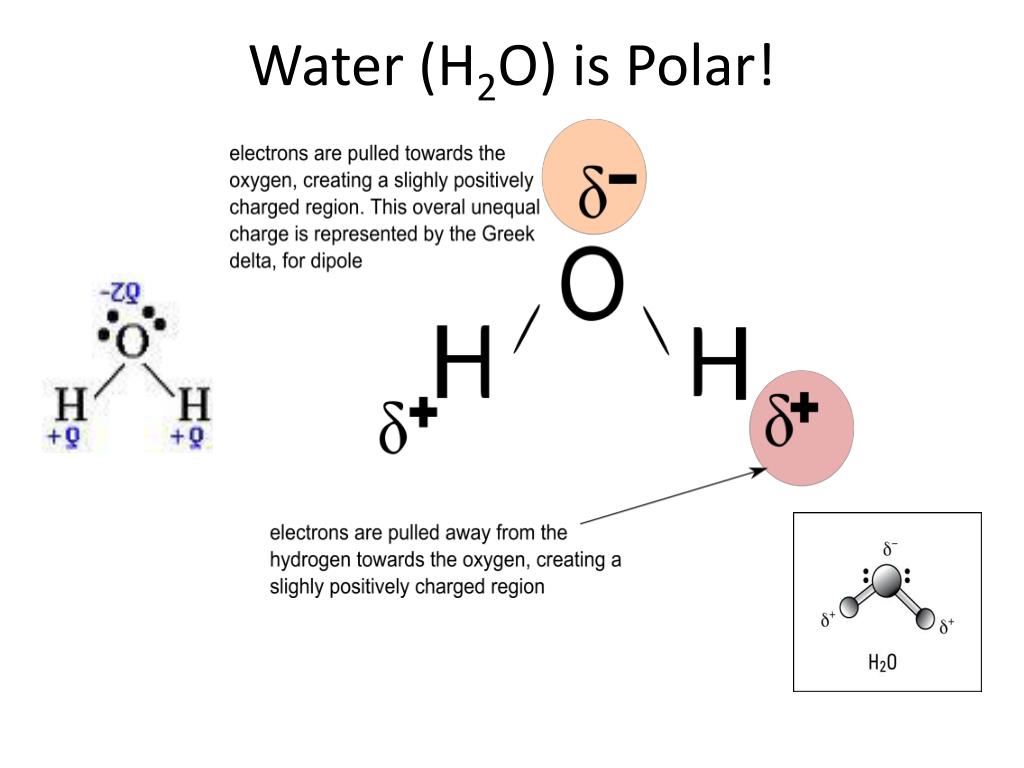
A molecules polarity rises from the electronegativity of the atoms in the molecule and the spatial positioning of the atoms. In other words, a polar bond forms. In a polar bond, one atom has a partial positive electrical charge, while the other atom has a partial negative electrical charge.
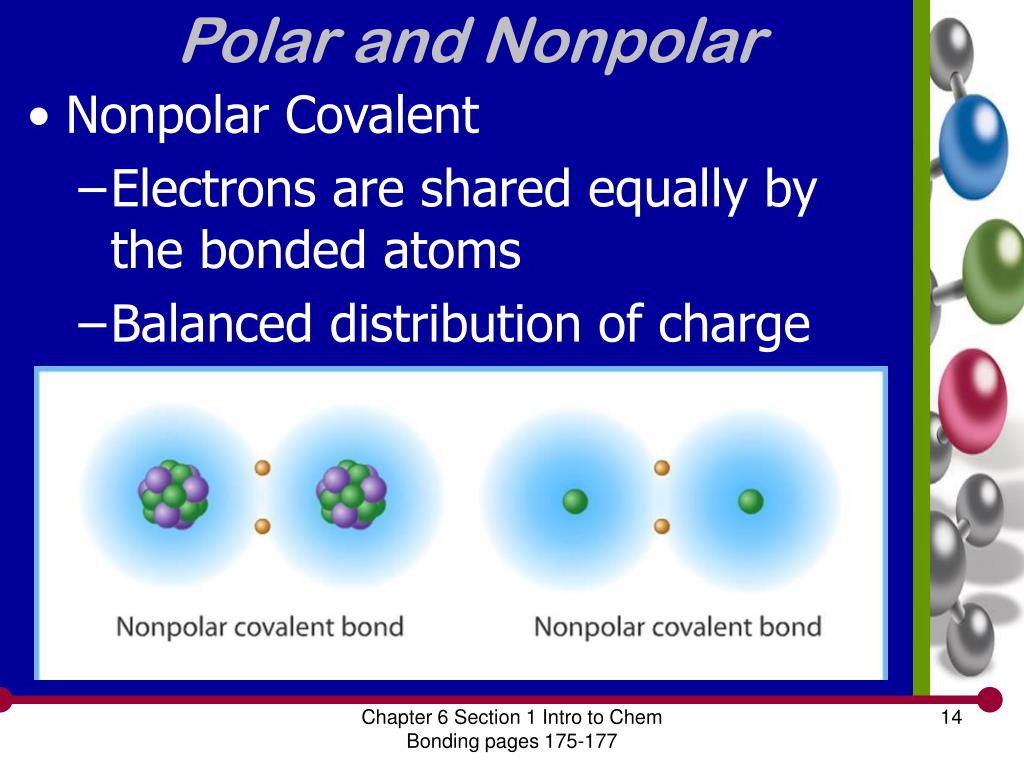
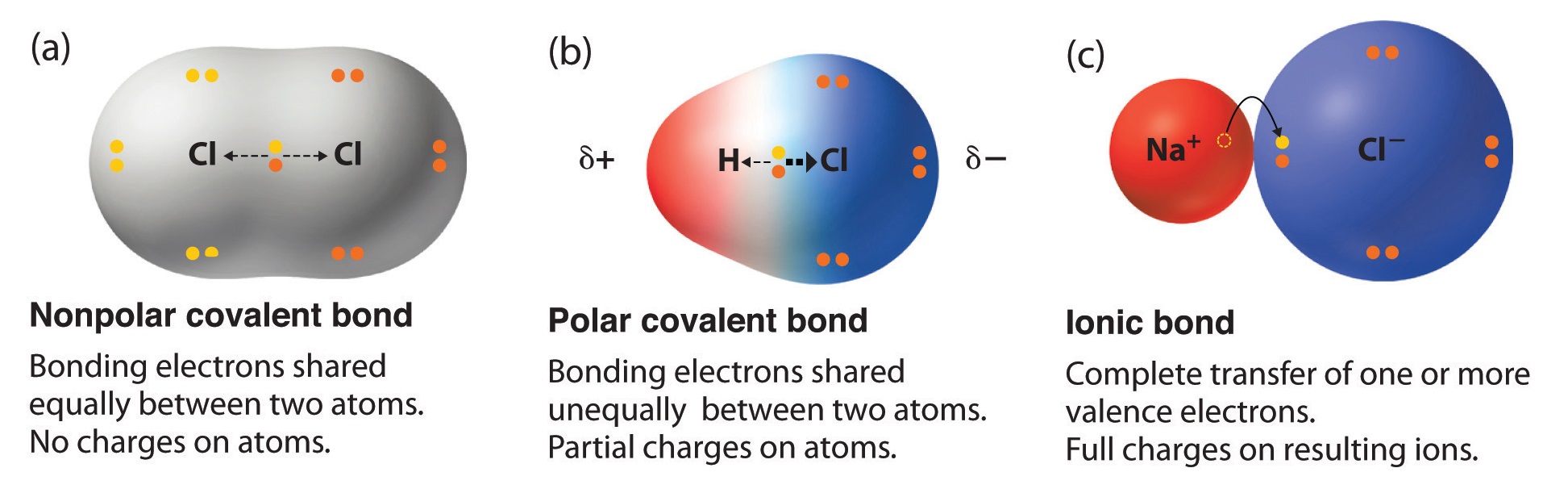
Polar molecules occur when two atoms do not share electrons equally in a covalent bond. The negative charge of electrons balances the positive charge of protons in an atom. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge.
Figure 4.4.1 4.4. How to draw lewis structures: 4m views 10 years ago chemistry.
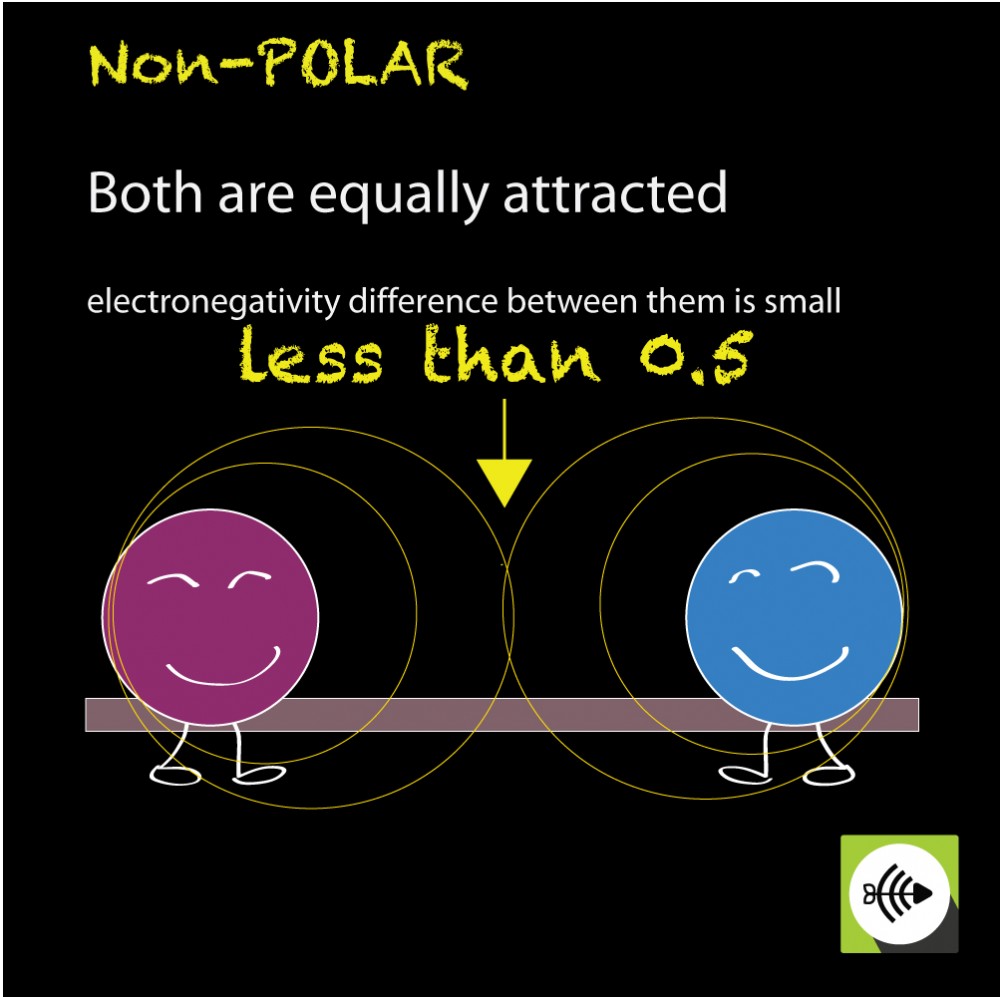
Generally, bonds between two of the same atoms, such as nitrogen (n2) or oxygen (o2) have an even. When atoms with various electronegativities share electrons to form a covalent bond, the result is a polar covalent bond. A completely polar bond occurs when one of the atoms is so electronegative that it takes an electron from the other atom (this is called an ionic bond.
This chemistry video tutorial provides a basic introduction into polar and nonpolar molecules. 1 polar versus nonpolar covalent bonds. To determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures.
Predict the structures of small molecules using valence shell electron pair repulsion (vsepr). This video discusses how to tell if a molecule / compound is polar or nonpolar. The electrons symmetrically distributed around the nucleus leave no.
In addition to determining whether a specific bond is polar or nonpolar, we can also consider how all of the bonds in a molecule combine to create polar and. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. This video provides a fast way for you to determine if a molecule is polar or nonpolar.
It provides examples so you can quickly distinguish nonpolar molecul. Polar covalent bonds. How to determine if a molecule is polar or nonpolar.